-
PDF
- Split View
-
Views
-
Cite
Cite
Ilona Kohler, Dietmar Jacob, Jan Budzies, Annika Lehmann, Wilko Weichert, Stefan Schulz, Peter Neuhaus, Christoph Röcken, Phenotypic and Genotypic Characterization of Carcinomas of the Papilla of Vater Has Prognostic and Putative Therapeutic Implications, American Journal of Clinical Pathology, Volume 135, Issue 2, February 2011, Pages 202–211, https://doi.org/10.1309/AJCPCTCUQSYI89YT
Close - Share Icon Share
Abstract
We further characterize the heterogeneous carcinomas of the papilla of Vater (CPVs) in relation to various clinicopathologic patient characteristics and patient survival.
Of the 71 reevaluated CPVs, 32 were intestinal, 26 were pancreatobiliary, 6 were mixed, 4 were mucinous, and 3 were poorly differentiated carcinomas. The prevalence of cytokeratin 20 and cytokeratin 7 correlated with the intestinal (25/32 [78%] vs 13/32 [41%]) and pancreatobiliary (6/26 [23%] vs 24/26 [92%]) phenotypes. CDX2 was found in mucinous (3/4 [75%]), intestinal (7/32 [22%]), and some mixed (1/6 [1%]) CPVs. A KRAS mutation was detected in all poorly differentiated CPVs and in about 20% of each of the other types. In multivariate analyses, tumor type, local tumor spread, and lymph node metastases were independent prognostic factors of patient survival.
We provide further evidence of the prognostic relevance of the phenotypic and genotypic diversity of CPVs. Besides the poorly differentiated CPV, the most common KRAS wild type makes them a putative target for an anti–epidermal growth factor receptor therapy.
Carcinomas of the papilla of Vater (CPVs) are a rare tumor entity that came into focus in recent years. The interesting feature of these carcinomas is their origin at the ampulla of Vater where different epithelia meet, ie, of the pancreatic duct, the bile duct, and the duodenal mucosa. The prognosis of the CPV probably pictures this heterogeneity. It differs considerably (5-year survival, 34%–66%) and lies between the survival of patients with pancreatic carcinoma (5-year survival, 20%) and patients with bile duct carcinoma or duodenal carcinoma (5-year survival, 74% or 85%).
These tumors are further characterized by variable histologic morphologic features. Kimura et al1 and Albores-Saavedra et al2 took this into account and introduced a histologic classification for these tumors, ie, pancreatobiliary, intestinal, mixed, mucinous, poorly differentiated, and invasive papillary types; signet ring cell carcinoma; and clear cell carcinoma. The histologic features of these tumors may be of paramount relevance to assess patient prognosis and tumor biology because the local tumor growth sometimes prohibits a clear assignment of the histoanatomic origin and, hence, pathogenesis of these lesions.
Although the alternative histologic classification was not “enforced” until now by the World Health Organization, previous studies showed the importance of the histologic distinction for patient prognosis. CPV of the pancreatobiliary type has a worse prognosis than CPV of the intestinal type.3,4 Moreover, the different histologic subtypes may be explained by the different tissue types they originate from, ie, the pancreas, the duodenum, or the bile duct.3 Only a few investigations5–7 tried to separate cases of CPV by the conventional histologic classification proposed by Kimura et al1 and Albores-Saavedra et al.2 However, in some cases, the differentiation may be difficult. Therefore, Fischer and Zhou5 supported histologic examination by immunohistochemical studies. They showed that the intestinal type of CPV typically expresses cytokeratin (CK)20 and the pancreatobiliary type, CK7. These results are in accordance with the known CK expression pattern of nonneoplastic epithelium of the intestinal mucosa (CK20) and pancreatobiliary epithelium (CK7) and further support the hypothesis that CPVs are of diverse origin. However, most of the aforementioned investigations did not consider all subtypes of the classification of Albores-Saavedra et al,2 but focused mainly on the intestinal and pancreatobiliary types. Apart from histologic features, the genotype of the CPVs, such as the KRAS genotype, is also of interest and may influence patient management in the future.
In this study, our aims were to fill these gaps of previous investigations and to assign 71 cases of CPV histologically into the different subgroups of the classification of Albores-Saavedra et al.2 Subsequently, we carried out immunohistochemical investigations, analyzed the KRAS genotype, and correlated the results with various clinicopathologic patient characteristics. We further provide evidence that the CPVs have distinct phenotypes and genotypes, which influence the patient’s prognosis and may be suitable to tailor patient management in the future.
Materials and Methods
Patient Characteristics
We retrospectively investigated 71 cases from the Campus Virchow Hospital, Charité University Hospital, Berlin, Germany, in which curative operation was done for a CPV between 1993 and 2007. The patients were between 29 and 83 years old (mean age, 61.8 years); 43 were men, and 28 were women. Only cases with an adenocarcinoma with the main tumor mass in and around the papilla of Vater were included. Other origins, eg, carcinoma of the distal bile duct or pancreatic carcinoma, were excluded. Formalin-fixed and paraffin-embedded tissue samples of the tumor and extralesional tissue from all cases were retrieved from the archive.
Survival data were obtained for 65 patients; 38 patients died during the follow-up period. The cutoff date for follow-up was July 1, 2007.
Histologic Classification of Surgical Pathology Specimens
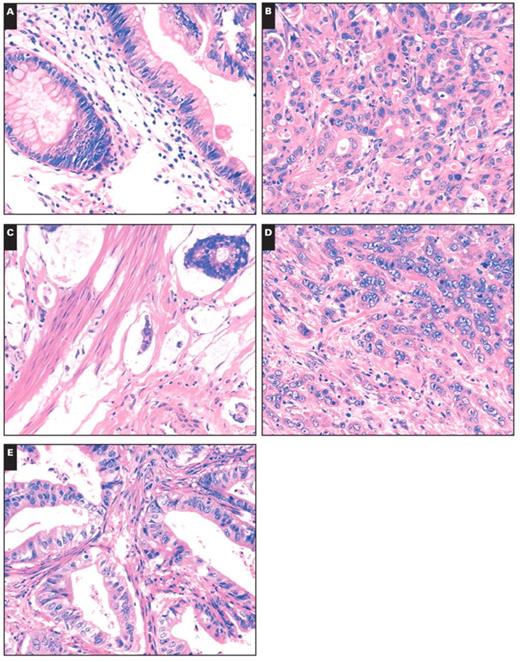
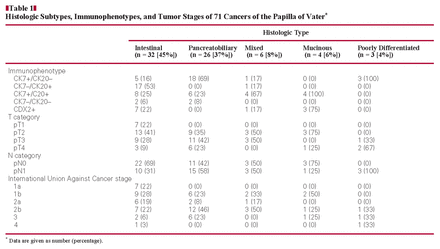
Two pathologists (I.K. and C.R.) separately evaluated the histologic features of all cases using H&E-stained sections on which the original diagnosis was made. The evaluation was performed according to the classification of Albores-Saavedra et al,2 which separated the tumors into intestinal, pancreatobiliary, mixed, poorly differentiated, mucinous, and invasive papillary types and signet ring cell and clear cell carcinoma types Image 1. The intestinal type consists of tubular or cribriform glands with columnar cells and pseudostratified nuclei. The pancreatobiliary type shows characteristics of pancreatic carcinoma with simple or branching glands that are lined by cuboidal to low columnar cells with rounded nuclei. The mixed type showed characteristics of the intestinal and pancreatobiliary types. Poorly differentiated carcinomas consist of solid sheets or nests with marked nuclear atypia. The invasive papillary carcinoma is characterized by papillary formations of the invasive component. Tumor stage was assessed according to the guidelines set by the International Union Against Cancer.
Tissue Microarray Construction
Formalin-fixed and paraffin-embedded tissue samples were used to generate tissue microarrays (TMAs). Briefly, morphologically representative regions of the paraffin “donor” block were chosen, ie, the tumor, the nonneoplastic duodenal mucosa, and the nonneoplastic common bile duct were marked on the respective H&E-stained slides. Tissue cylinders were punched from these areas and precisely arrayed into a new “recipient” paraffin block using a commercially built instrument (Beecher Instruments, Silver Spring, MD). Three tissue cylinders of 0.6-mm diameter were punched from each region. Tumor was available in all 71 cases. Tissue from the duodenal mucosa was available in 70 cases and from the common bile duct in 64 cases. After completing the block construction, 4-μm sections of the resulting TMA block were cut for further analysis. Successful tissue transfer was assessed on H&E-stained sections obtained from the TMA.
Immunohistochemical Analysis
Immunohistochemical analysis was carried out with commercially available antibodies directed against CK7, CK20, and CDX2 (all DAKO, Carpinteria, CA).
The Ventana Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ) was used for immunostaining with the anti-CK7 antibody (dilution 1:1,000), the anti-CK20 antibody (dilution 1:100), and the anti-CDX2 antibody (dilution 1:25). The specimens were counterstained with hematoxylin. Omission of primary antibodies served as negative controls.
Analysis of the KRAS Genotype
DNA Preparation
Three consecutive paraffin sections from tumor blocks were used to extract DNA. Tumor-bearing areas were micro-dissected from regions corresponding to a fourth H&E-stained tissue section. DNA preparation was carried out as previously described.8 For DNA preparation, microdissected tissue was transferred to 180 μL ATL buffer (Qiagen, Hilden, Germany) and kept for 10 minutes at 95°C. After cooling to room temperature, 20 μL of Proteinase K solution was added. The sample was gently mixed and incubated at 55°C until complete lysis was achieved (after about 2 hours). Further steps of DNA isolation were performed according to the protocol “Tissue Protocol-QIAamp DNA Mini Kit” (Qiagen). The nucleic acid was eluted in a volume of 60 to 100 μL, and DNA content was measured with a Nanodrop 1000 spectrophotometer (PeqLab, Erlangen, Germany).
Histologic subtypes of carcinoma of the papilla of Vater. A, Intestinal. B, Pancreatobiliary. C, Mucinous. D, Poorly differentiated. E, Mixed (A–E, H&E, ×200).
Pyrosequencing
Preparation for the sequencing reaction was done using the PyroMark KRAS Kit (Biotage, Uppsala, Sweden), according to the manufacturer’s instructions. In brief, 10 ng of genomic DNA, as well as forward and reverse KRAS polymerase chain reaction (PCR) primers were used for PCR amplification of the region containing codons 12 and 13. PCR cycling conditions were as follows: 95°C for 15 minutes; 45 cycles of 95°C for 50 seconds and 58°C for 50 seconds, and 72°C for 50 seconds and 72°C for 10 minutes. Single-strand preparation of PCR products was done by immobilization on streptavidin-coated Sepharose beads (Amersham Biosciences, Uppsala, Sweden), using a Vacuum Prep Tool (Biotage). After adding specific sequencing primers (PyroMark KRAS Kit), samples were run on a PSQ 96 MA Pyrosequencer (Biotage) and subsequently analyzed by PSQ 96MA SNP/Pyromark ID software (Biotage).
Statistical Analysis
Statistics were done with SPSS version 15.0 (SPSS, Chicago, IL). The Pearson coefficient was assessed for correlation of the immunoprofile with clinicopathologic parameters. The different clinicopathologic characteristics, such as local spread of tumor or lymph node metastases, were used as variables in the χ2 test for trends. Survival curves were generated with the Kaplan-Meier method, and differences in survival were assessed by using the log rank test. Multivariate survival analyses were carried out using the Cox proportional hazard models. P values less than .05 were considered statistically significant. The Cohen κ was used for calculation of reliability for both pathologists.
Results
Histologic and Immunohistologic Classification
Two pathologists (I.K. and C.R.) classified 90% of the collective samples (64/71) independently in the same histologic group, for a κ value of 84.3%. In 7 cases (10%), consensus classification was needed. Five of these tumors were discussed to be a mixed or pancreatobiliary type. The sixth and seventh cases were categorized as poorly differentiated or intestinal and as pancreatobiliary or intestinal, respectively.
Finally, consensus was reached and 32 of the 71 CPVs were classified as intestinal, 26 as pancreatobiliary, 6 as mixed, 4 as mucinous, and 3 as poorly differentiated, as shown in Table 1. No signet ring cell carcinoma, invasive papillary carcinoma, or clear cell carcinoma was identified.
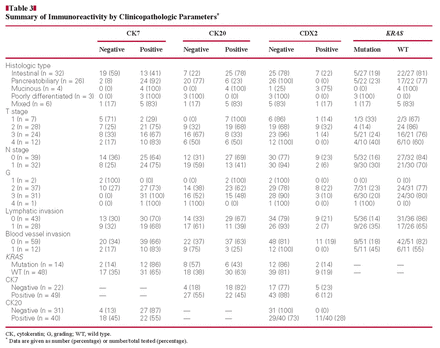
Histologic Subtypes, Immunophenotypes, and Tumor Stages of 71 Cancers of the Papilla of Vater*
Histologic Subtypes, Immunophenotypes, and Tumor Stages of 71 Cancers of the Papilla of Vater*

Immunophenotype
Cytokeratins
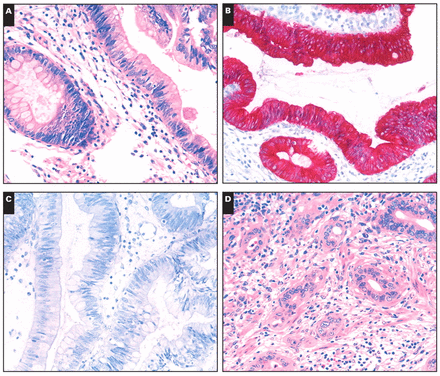
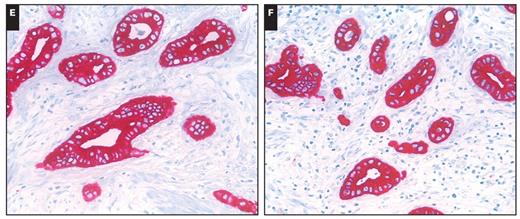
The CK7 and CK20 phenotypes of the CPVs are summarized in Table 1. Overall 25 (78%) of the intestinal-type cancers expressed CK20 compared with 6 (23%) of the pancreatobiliary-type cancers (P < .001). In contrast, the pancreatobiliary type significantly more commonly expressed CK7 (24 [92%]; P < .001). A mixed CK7+/CK20+ phenotype was found in 6 (23%) of the pancreatobiliary-type and 8 (25%) of the intestinal-type cases Image 2. Thus, the CK expression pattern correlated with the histologic phenotype.
The cytokeratin expression pattern was less distinctive in the other 3 phenotypes. Of the 6 mixed-type cancers, 4 (67%) coexpressed CK7 and CK20. Coexpression was subsequently verified on large tissue sections of the tumor-bearing paraffin blocks obtained from all 6 cases. All 4 mucinous-type carcinomas (100%) were immunoreactive for CK7 and CK20. It is interesting that all 3 poorly differentiated carcinomas (100%) expressed only CK7.
CDX2 Immunoreactivity
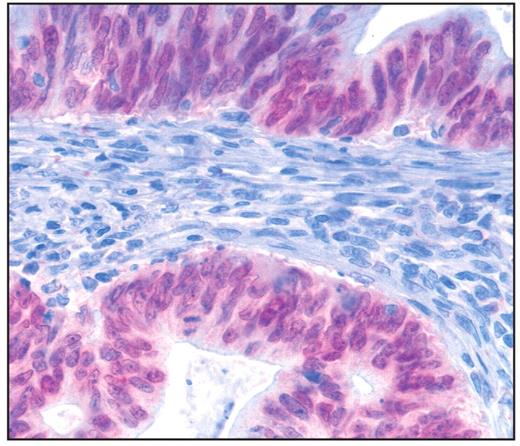
CDX2 was expressed in the tumor cell nuclei of 7 (22%) of the intestinal type, 3 (75%) of the mucinous type, and 1 (17%) of the mixed type Image 3. None of the pancreatobiliary type or the poorly differentiated type was immunoreactive for CDX2. For statistical analysis by the χ2 test, the mucinous, mixed, and pancreatobiliary types were grouped. The analysis confirmed the obvious relation of CDX2 immunoreactivity to the histologic subtype (P = .05). A trend of an inverse correlation was calculated for CDX2 immunoreactivity to the T and N stages (r = −0.28 and r = −0.23, respectively). Moreover, tumors seemed to express CDX2 and CK20 (r = 0.38). No correlation was found with the stages of lymphatic and blood vessel invasion, grading, or CK7 immunoreactivity (r = −0.12, r = −0.19, r = −0.12, and r = −0.13, respectively) Table 2 and Table 3.
Immunophenotype of intestinal (A, B, and C) and mixed (D, E, and F) types of carcinomas of the papilla of Vater (A and D, H&E, ×200; B and E, anti-cytokeratin 20 antibody, H&E, ×200; C and F, anti-cytokeratin 7 antibody, H&E, ×200).
Expression of CDX2 in carcinomas of the papilla of Vater. Note the nuclear reaction of CDX2 in an intestinal-type cancer (×400).
KRAS Genotype
KRAS genotyping was possible in the tumor samples obtained in 62 cases (87%). In 9 cases (5 intestinal and 4 pancreatobiliary), the tumor samples did not provide a sufficient quality of DNA for genotyping.
Summary of Correlation (r) of Clinicopathologic Parameters and Immunoreactivity of CK7 and CK20 to the Reaction of CDX2 or the Status of KRAS
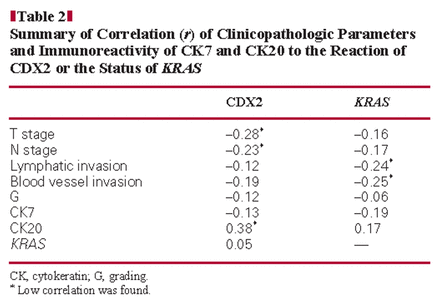
Summary of Correlation (r) of Clinicopathologic Parameters and Immunoreactivity of CK7 and CK20 to the Reaction of CDX2 or the Status of KRAS

A KRAS mutation in codon 12 or 13 was found in 14 cases (23%) and was assigned to 5 intestinal (36%), 5 pancreatobiliary (36%), 3 poorly differentiated (21%), and 1 mixed (7%) type. Correlation of the prevalence of a KRAS-mutation with histologic phenotype showed that the KRAS mutation was most prevalent in the poorly differentiated type (3/3 [100%]), followed by the pancreatobiliary (5/22 [23%]), intestinal (5/27 [19%]), and mixed (1/6 [17%]) types, but these results did not reach statistical significance. The distribution of the individual KRAS mutations is summarized in Table 4.
A trend toward a negative correlation was found between the KRAS mutation status and the stages of lymphatic and blood vessel invasion (r = −0.24 and r = −0.25, respectively). No correlation was found to the T stage, N stage, or grade (r = −0.16, r = −0.17, and r = −0.06, respectively) or to the immunoreactivity of CK7, CK20, and CDX2 (r = −0.19, r = 0.17, and r = 0.05 respectively) (Tables 2 and 3).
Prevalence of KRAS Mutations in Histologic Subtypes of Carcinomas of the Papilla of Vater*

Prevalence of KRAS Mutations in Histologic Subtypes of Carcinomas of the Papilla of Vater*

Clinicopathologic Characteristics
The correlation of histologic type of CPV with the TNM stage is shown in Table 1. Comparison of the pancreatobiliary with the intestinal type revealed that the former showed more advanced local tumor spread (P < .01). Similarly, pancreatobiliary-type cancers had significantly more commonly metastasized to lymph nodes than had intestinal-type CPVs (P < .05). No correlation of histologic subtype to lymphatic (P = .43) and blood vessel invasion (P = .74) was found.
Survival
Survival data were available for 65 patients. The longest mean survival periods were in patients with intestinal-type carcinoma, with 2,777 days, and mixed-type carcinoma, with 1,880 days. Patients with pancreatobiliary-type carcinoma lived on average 804 days and with a mucinous-type carcinoma, 564 days. The poorly differentiated type carcinoma revealed the shortest mean survival, 85 days.
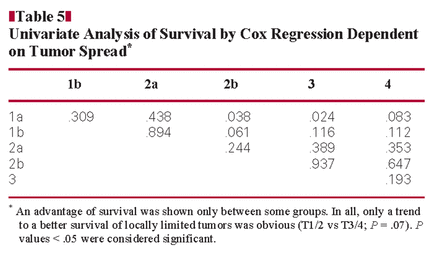
First, we correlated patient survival with the TNM stage independent of the histologic type in univariate analysis. The overall patient prognosis correlated with lymphatic invasion (L category; P = .012) and nodal spread (N category; P = .008). The local tumor growth showed a trend to correlate with the prognosis (T category; T1/2 vs T3/4, P = .07). The subgroup analysis of the T stage showed a significant advantage of survival in some of the stages (T1a vs T2b, P = .038 and T1a vs T3, P = .024) Table 5. CK7 seemed to influence patient prognosis, too (P = .044). The analysis of all other markers, CK20, CDX2, and KRAS, did not reach significance (P = .53, P = .94 and P = .28, respectively).
Univariate Analysis of Survival by Cox Regression Dependent on Histologic Type*
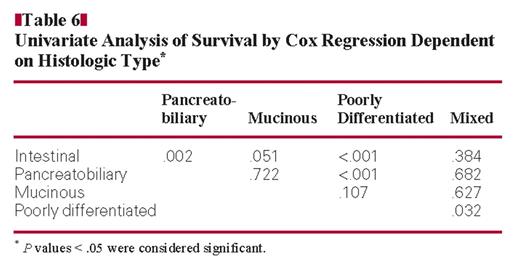
Univariate Analysis of Survival by Cox Regression Dependent on Histologic Type*

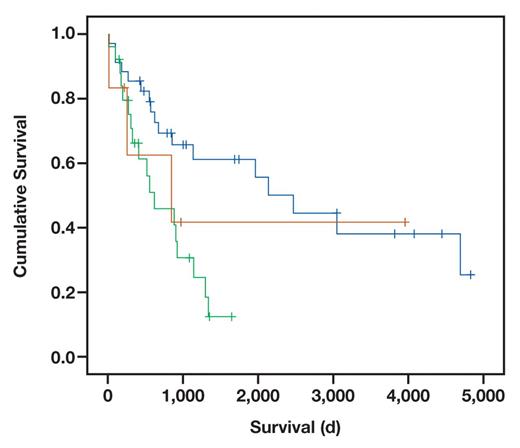
Next, we correlated patient prognosis with the histologic type Table 6. Patients with the pancreatobiliary type had a significantly worse prognosis than patients with an intestinal type (P = .002) Figure 1. When analyzing the remaining subgroups, the small patient numbers have to be kept in mind. Nevertheless, differences in patient survival were obvious. Patients with an intestinal type had a better prognosis than patients with a poorly differentiated type.
Finally, we carried out a probatory multivariate analysis including intestinal-type CPV, pancreatobiliary-type CPV, and the T, lymphatic invasion, and N categories. Tumor type (P = .01), local tumor spread (P = .017), and lymph node metastases (P = .017) were independent predictors of patient survival.
Discussion
CPV is a rare tumor, and only a limited number of studies have been published on this tumor entity. Kimura et al1 and Albores-Saavedra et al2 proposed a histologic classification of CPVs that may have prognostic and therapeutic implications for patients.
Kaplan-Meier curve for survival depending on histologic subtypes. Survival was significantly better in the intestinal-type carcinoma of the papilla of Vater (blue line) than the pancreatobiliary type (green line) (P = .002). The third group (brown line) includes the remaining carcinomas (mucinous, mixed, and poorly differentiated). The short crossed lines mark censored cases.
Our retrospective investigation of 71 CPVs operated on between 1993 and 2007 confirms the applicability of the classification system and the histologic variability of CPVs: the diagnoses of 2 pathologists were concordant in 90% of the cases. In the remaining cases, classification was somewhat more difficult because these tumors often showed a greater variability of the phenotype, and, hence, a consensus was reached to classify these tumors as mixed, pancreatobiliary, or intestinal.
The prevalences of the different subtypes observed in our series are also in line with observations made by others. The overall prevalence of the intestinal type ranged from 27% to 49% and of the pancreatobiliary type from 21% to 45%.2,3,5,9 However, only limited information is available on the other types of CPV. The first reports of more than 2 histologic types published by Albores-Saavedra et al2 classified 4 poorly differentiated, 9 invasive papillary, 15 mucinous, and 3 signet-ring cell carcinomas. Carter et al3 found 4 mucinous signet-ring carcinomas, 1 carcinosarcoma, and 6 indeterminate cancer types in 118 patients with ampullary adenocarcinoma, and Chu et al9 found 1 mucinous, 3 signet-ring cell carcinomas, and 1 mixed type in 18 patients with adenocarcinoma of the ampulla of Vater. The newest and largest investigation described 22 poorly differentiated, 14 mucinous, and 9 invasive papillary carcinomas; 1 each noninvasive papillary carcinoma, signet-ring cell carcinoma, and neuroendocrine carcinoma; and 3 nonclassifiable carcinomas in 170 periampullary carcinomas,7 while we classified 6 mixed, 4 mucinous, and 3 poorly differentiated cancers.
The phenotypic diversity of the conventional histologic findings was then validated by immunohistochemical analysis. The CK7 and CK20 expression patterns correlated with the pancreatobiliary and intestinal phenotypes. The tumors that were difficult to categorize and had to be discussed for consensus mostly showed a mixed immunophenotype. In this respect, it is interesting to note that all poorly differentiated carcinomas expressed only CK7. This leads to the conjecture that poorly differentiated carcinomas may stem from the pancreatobiliary-type cancer. Similarly, the immunophenotype of mucinous-type cancers was similar to that of mixed-type cancers, which may also be interpreted in a way that mixed-and mucinous-type cancers are 2 extremes of 1 subgroup. This observation supplies the recent assumption that the different subtypes originate from the distinct epithelium converging at the papilla of Vater. Therefore, the histologic features may not only be important for prognosis but may also have an important role for therapeutic considerations of whether intestinal-type carcinomas should be treated like colorectal cancer and pancreatobiliary-type carcinomas like pancreatic cancer. We tried to confirm these impressions of totally different characteristics of CPV by further analyses.
Adenocarcinomas of the colorectum commonly express the parahomeobox gene cdx2, which is also used as a general diagnostic marker of intestinal-type adenocarcinomas.10,11 Our aim was to test the hypothesis whether intestinal-type CPVs share similarities with colorectal cancers (CRC) by expressing CDX2. In line with CK20 positivity, CDX2 was mainly found in the intestinal-type (22%) compared with the pancreatobiliary type (0%), concordant to recent studies by Chu et al9 and Sessa et al.12 However, the prevalence of CDX2 in intestinal-type CPVs was far lower than expected. Several studies reported a prevalence of CDX2 in CRC reaching 50% to 90%, which is 4 times more frequent.10,13,14 Hansel et al15 found a significant correlation between the expression of CDX2 and patient survival in CPVs. While we were unable to find a direct correlation between CDX2 and patient survival in our own series, our observations are still in line with the findings of Hansel et al.15 In our series, CDX2 was more prevalent in intestinal-type CPVs, which had a significantly better prognosis, than in pancreatobiliary-type CPVs. Despite similar morphologic features and CK immunoprofile, intestinal-type CPVs may be different from CRC.
To further substantiate this difference, we carried out KRAS genotyping and found KRAS mutations in only 19% of the intestinal type, which is 50% less than in CRC. Thus, the prevalence of the CDX2 immunophenotype and KRAS genotype is dissimilar from CRC, suggesting that this is a distinct tumor entity.
KRAS genotyping also substantiated the differences of CPVs from typical ductal adenocarcinomas of the pancreas, which is believed to range between 49% and 90%.16–18 Only the poorly differentiated carcinomas showed a high prevalence of KRAS mutations, and because all of these tumors also expressed CK7, they may indeed be more similar to the typical ductal adenocarcinoma of the pancreas than to all other CPV variants.
Two research groups reported prevalence rates of KRAS mutations in their series of CPVs of 44% and 28.6%.19,20 We cannot fully explain the different prevalence rates of KRAS mutations. Our KRAS testing passed 2 external quality assurance programs successfully, and we dissected the tumor cells before KRAS testing. Thus, a sampling error and methodological problems are highly unlikely.
The low prevalence of KRAS mutations is also interesting with regard to putative therapeutic implications. Intestinal-and pancreatobiliary-type CPVs commonly harbor wild-type KRAS and, hence, may be suitable for treatment with epidermal growth factor receptor–targeted therapy with cetuximab or panitumumab. Further studies on this topic are warranted.
Finally, we correlated the various clinicopathologic parameters of our CPV collective with histologic subtype and patient prognosis. The different survival of the histologic subtypes shown by Westgaard et al4 and Carter et al3 was confirmed in our collective. Patients with the intestinal type survived significantly longer than patients with the pancreatobiliary type. Owing to the small number of cases, the other 3 histologic groups were not included in this analysis. Nevertheless, the raw survival times differed clearly. Whereas the intestinal type had the longest survival, with 2,777 days, survival with the pancreatobiliary type was better than the mucinous type (804 vs 564 days) and the poorly differentiated type (85 days). The survival time of the mixed type was between the intestinal and the pancreatobiliary type, with 1,880 days, corresponding to a mixture between both histologic types. The very short survival time with the poorly differentiated type is, despite the small group, striking and may be explained by more aggressive tumor behavior and, therefore, a worse prognosis.
Besides the histologic features, lymph node metastases and the local tumor growth proved to be independent factors for survival, as also shown by other research groups.3,5,12,21–26 Despite the correlation of the pancreatobiliary type with local tumor spread, the histologic subtype was the best marker for prognosis in multivariate analysis and should, therefore, be used to tailor patient management.
Our study provides further evidence that CPVs are a heterogeneous group of cancers, which differ by conventional histologic features, immunophenotype (CK7, CK20, and CDX2), and KRAS genotype from each other and also from ductal carcinomas of the pancreas and CRC. The differences have an influence on patient prognosis, while a high prevalence of KRAS wild type may make these tumors particularly interesting for epidermal growth factor receptor–targeted therapy. We showed that the correct histologic diagnosis is not only important for the prognosis of the patients but also may have therapeutic consequences. Future studies should take this diversity into account. We hypothesize that these tumors necessitate highly patient- and tumor-individualized therapy.
Upon completion of this activity you will be able to:
distinguish the most frequent types of carcinoma of the papilla of Vater.
discuss the value of recognizing and reporting differentiation of the distinct types of carcinoma of the papilla of Vater.
compare the different immunohistologic patterns of the most frequent histologic types of carcinomas of the papilla of Vater.
discuss the different types of KRAS mutation status of carcinomas of the papilla of Vater.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit ™ per article. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 310. Exam is located at www.ascp.org/ajcpcme.
References
Author notes
Drs Kohler and Jacob shared first authorship.