-
PDF
- Split View
-
Views
-
Cite
Cite
Wang Xingfu, Zhang Lifeng, Chen Yupeng, Liu Xueyong, Liu Wei, Yu Yinghao, Cai Suqin, Wang Mi, Zhang Sheng, Cytoplasmic 5-Lipoxygenase Staining Is a Highly Sensitive Marker of Human Tumors of the Choroid Plexus, American Journal of Clinical Pathology, Volume 144, Issue 2, August 2015, Pages 295–304, https://doi.org/10.1309/AJCPMAIAATN88OJA
Close - Share Icon Share
Abstract
To determine the immunoreactivity status of 5-lipoxygenase (5-LO) in normal tissues, in tumors of the human choroid plexus, and in other brain tumors.
In total, 135 cases of various types of brain tumors were selected. Tissue samples were immunostained with a rabbit polyclonal anti–5-LO antibody.
Nuclear reactivity was observed in most brain tumors, with most of the positive tumor cells exhibiting low-level reactivity. Cytoplasmic strong immunoreactivity for 5-LO (2+ or 3+) was only observed in 8.8% of astrocytic tumors, 0% of oligodendrogliomatous tumors, 5.6% of ependymal tumors, 0% of embryonal tumors, 3.1% of meningeal tumors, and 0% of metastatic lung adenocarcinomas. In contrast, cytoplasmic immunoreactivity for 5-LO was detected in all 27 cases of choroid plexus tumors. Twenty-five cases showed strong and diffuse cytoplasmic immunoreactivity.
Our findings indicate that cytoplasmic 5-LO immunoreactivity is highly characteristic of human choroid plexus tumors but not other central nervous system tumor types. Cytoplasmic staining for 5-LO may prove to be a useful immunoreactive marker in the diagnosis of choroid plexus tumors.
5-Lipoxygenase (5-LO) is the key enzyme involved in the synthesis of proinflammatory leukotrienes (LTs) and is a mediator of inflammation that has been linked to carcinogenesis.1 A small number of studies have recently suggested that 5-LO pathway metabolites are involved in the progression of various types of cancer, including prostate cancer,2,3 pancreatic cancer,4 esophageal cancer,5 hepatocellular carcinoma,6 and papillary thyroid carcinoma.7 Recent studies have reported the strong immunoreactivity of 5-LO in Purkinje neurons, certain glial cells in the cerebellum, and parts of brain tumors.8,9 Currently, no reports have been made on the immunoreactivity status of 5-LO in normal and neoplastic tissues of the human choroid plexus. One study reported that most cells in the choroid plexus of mouse brain express LTCS, an important downstream metabolite of 5-LO.10 We observed strong cytoplasmic immunoreactivity of 5-LO protein as an incidental finding in damaged choroid plexus tissue of experimentally hypoxic rats (data not shown). In response to this peculiar finding, we undertook an in-depth study of the immunoreactivity of 5-LO in normal tissue, in tumors of the human choroid plexus, and in other brain tumors.
Material and Methods
This study was approved by the local ethics committee of Fujian Medical University. For the study, 131 cases of primary brain tumors and four cases of cerebral metastases were selected from our database. Among 135 cases of tumor selected, choroid plexus tumors and atypical teratoid/rhabdoid tumors were selected during September 2008 to June 2014; the other tumors were selected from June 2013 to June 2014. Formalin-fixed, paraffin-embedded tumor samples were collected from the Department of Pathology of the First Affiliated Hospital of Fujian Medical University, China. The tumors were diagnosed according to the World Health Organization (WHO) criteria.11 The immunoreactivity of 5-LO was determined in the following tumors: astrocytic tumors (n = 34), including pilocytic astrocytoma (WHO grade I, n = 5), diffuse astrocytoma (WHO grade II, n = 7), anaplastic astrocytoma (WHO grade III, n = 7), and glioblastoma (WHO grade IV, n = 15); oligodendrogliomatous tumors (n = 10), including oligodendroglioma (WHO grade II, n = 6) and anaplastic oligodendroglioma (WHO grade III, n = 4); ependymal tumors (n = 18), including myxopapillary ependymoma (WHO grade I, n = 3), ependymoma (WHO grade II, n = 10; eg, classic, tanycytic types), and anaplastic ependymoma (WHO grade III, n = 5); tumors of the choroid plexus (n = 27), including choroid plexus papilloma (CPP) (WHO grade I, n = 19), atypical CPP (WHO grade II, n = 6), and choroid plexus carcinoma (CPC) (WHO grade III, n = 2); embryonal tumors (n = 10), including medulloblastoma (n = 8) and atypical teratoid/rhabdoid tumor (n = 2); meningeal tumors (n = 32), including meningioma (WHO grade I, n = 22; eg, meningotheliomatous, fibroblastic, transitional types), atypical meningiomas (WHO grade II, n = 8), and anaplastic meningioma (WHO grade III, n = 2); and cerebral metastatic lung adenocarcinoma (n = 4), including metastatic papillary adenocarcinoma (n = 1) and solid adenocarcinoma with mucin production (n = 3) Table 1.
Score Values of 5-Lipoxygenase Immunoreactivity in Primary and Metastatic Central Nervous System Tumors
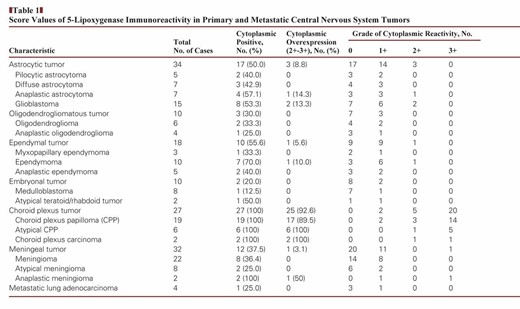
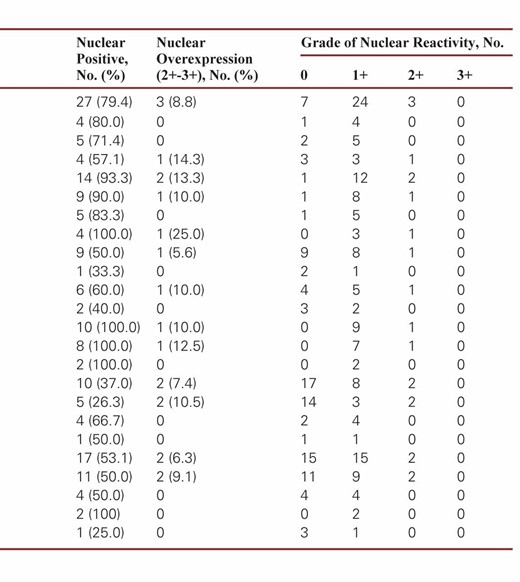
Score Values of 5-Lipoxygenase Immunoreactivity in Primary and Metastatic Central Nervous System Tumors


All samples were immunostained with a rabbit polyclonal anti–5-LO antibody (ab39347, 1:200 dilution; Abcam, Cambridge, England). To carry out the staining, 4-μm–thick sections were deparaffinized and rehydrated prior to antigen retrieval, which was performed by autoclaving samples in a 10-mmol/L citrate buffer (pH 6.0) for 3 minutes at 120°C. All tissue samples were then exposed to 3% hydrogen peroxidase for 5 minutes (to block endogenous peroxidase reactivity), primary antibody for 25 minutes, biotinylated secondary antibody for 25 minutes, the chromogen diaminobenzidine for 5 minutes, and a hematoxylin counterstain for 5 minutes. All immunohistochemical staining was performed using an automatic stainer (Kyowa Hakko, Tokyo, Japan). The stained slides were then coverslipped using the Tissue-Tek SCA automatic coverslipper (Sakura Finetek, Tokyo, Japan). All samples were fixed in 10% neutral buffered formalin overnight and embedded in paraffin, and they were immunostained under the same condition with the same batch of 5-LO antibody. The validity of the 5-LO staining was confirmed using a positive control (lymph node tissue samples). All the immunostained sections were evaluated by two senior pathologists (W.X. and Z.S.).
5-LO immunoreactivity was then scored according to the nuclear-positive and cytoplasmic-positive patterns. The cytoplasmic staining of 5-LO in tumor cells was evaluated in terms of the extent and intensity of immunoreactivity and classified on a 4-point scale: 0 (no labeling or labeling in <10% of tumor cells), 1+ (weak labeling, homogeneous or patchy, in >10% of tumor cells), 2+ (moderate labeling, homogeneous or patchy, in >10% of tumor cells), and 3+ (intense labeling, homogeneous or patchy, in >10% of tumor cells). The nuclear reactivity of 5-LO was scored as a percentage of tumor cells on a four-tier grading scheme (0, <1%; 1+, 1%–10%; 2+, 10%–25%; and 3+, >25%).
Results
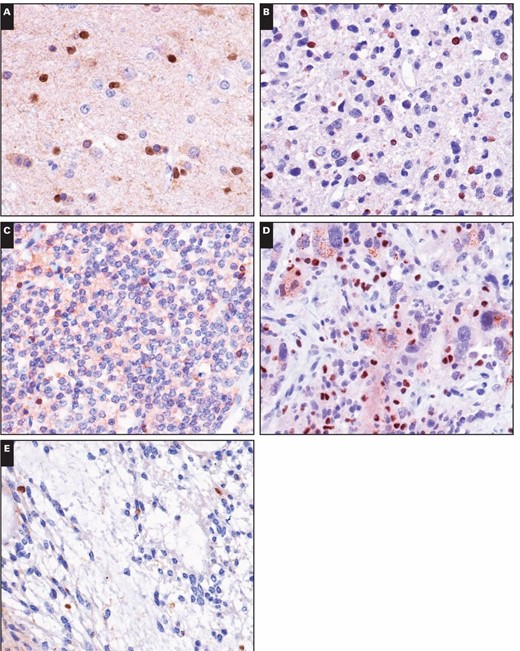
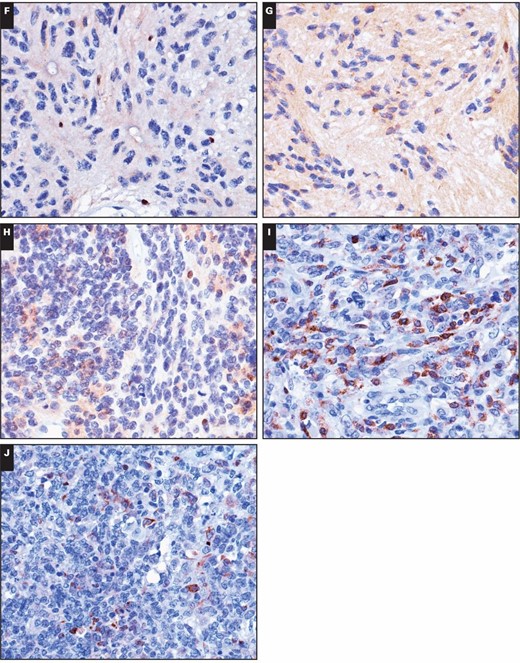
In our study, the two immunoreactivity patterns for 5-LO in brain tumors were nuclear staining and cytoplasmic staining. Nuclear reactivity was observed in most brain tumors, including astrocytomas, oligodendrogliomas, ependymomas, embryonal tumors, meningiomas, and metastatic lung adenocarcinomas, which showed immunoreactivity rates of 79.4%, 90.0%, 50.0%, 100%, 53.1%, and 25.0%, respectively. Regardless of histologic type, these non–choroid plexus neoplasms had mostly low levels (1+) of nuclear immunoreactivity, with only a relative few with higher levels (2+ or 3+) in three astrocytomas, one oligodendroglioma, one ependymoma, one medulloblastoma, and two meningiomas Image 1. Only two choroid plexus tumors had 2+ or greater nuclear immunoreactivity, and 25 had none. There was no significant difference in the nuclear strong immunoreactivity rate of 5-LO between tumors of the choroid plexus and other brain tumors (P > .05). These results are summarized in Table 1.
Immunohistochemical stains for 5-lipoxygenase (5-LO) in central nervous system tumors. Weak to moderate nuclear immunoreactivity of 5-LO was detected in the following tumors (×400): (A) diffuse astrocytoma, (B) anaplastic astrocytoma, (C) oligodendroglioma, (D) glioblastoma, and (E) myxopapillary ependymoma. Immunohistochemical stains for 5-lipoxygenase (5-LO) in central nervous system tumors. Weak to moderate nuclear immunoreactivity of 5-LO was detected in the following tumors (×400): (F) classic ependymoma, (G) tanycytic ependymoma, (H) anaplastic ependymoma, (I) medulloblastoma, and (J) atypical teratoid/rhabdoid tumor. Immunohistochemical stains for 5-lipoxygenase (5-LO) in central nervous system tumors. Weak to moderate nuclear immunoreactivity of 5-LO was detected in the following tumors (×400): (K) fibrous meningioma, (L) meningothelial meningioma, (M) anaplastic meningioma, (N) cerebral metastatic lung papillary adenocarcinoma, and (O) cerebral metastatic lung solid adenocarcinoma with mucin production.
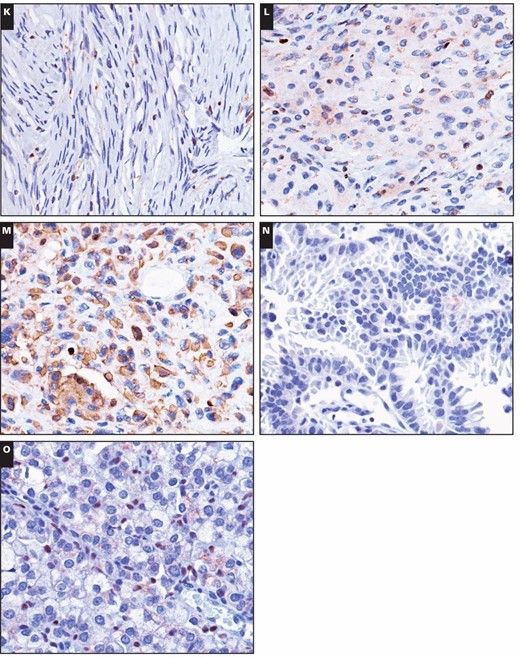
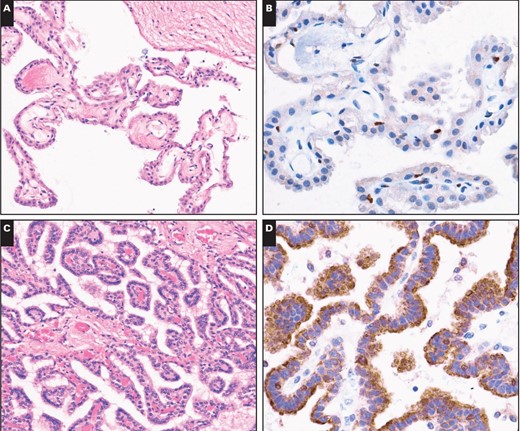
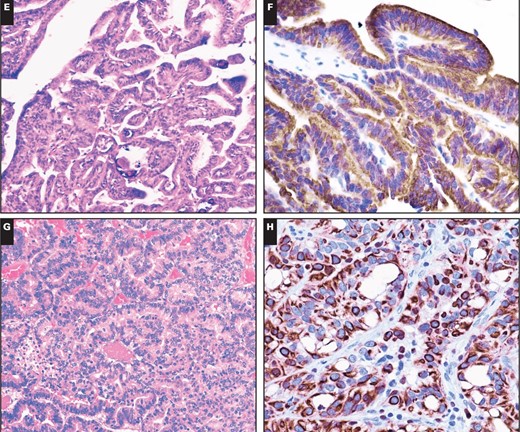
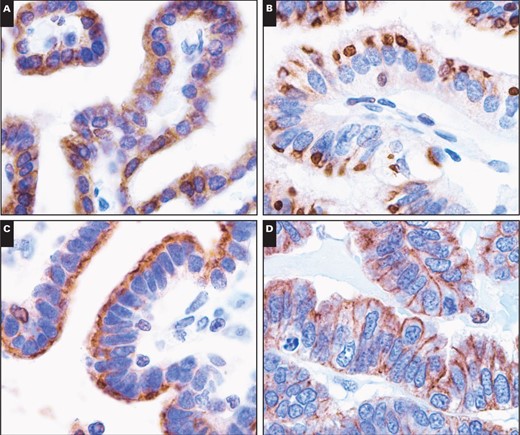
Cytoplasmic immunopositivity for 5-LO was observed in 50% of astrocytic tumors, 30% of oligodendrogliomatous tumors, 55.6% of ependymal tumors, 10% of embryonal tumors, 37.5% of meningeal tumors, and 25.0% of metastatic lung adenocarcinomas. However, stronger cytoplasmic immunopositivity of 5-LO (2+–3+) was observed in only 8.8%, 0%, 5.6%, 0%, 3.1%, and 0% of those respective tumor types. Among the samples showing strong immunopositivity, only one patient with anaplastic meningioma exhibited strong (3+) cytoplasmic immunoreactivity to 5-LO, and the others showed moderate staining. In contrast, cytoplasmic immunoreactivity for 5-LO was observed in all 27 cases of tumors from the choroid plexus Image 2C, Image 2D, Image 2E, Image 2F, Image 2G, and Image 2H. Twenty-five of the 27 cases showed strong and diffuse cytoplasmic immunoreactivity (2+–3+ intensity and extent) with a lack of nuclear immunoreactivity. The other two tumors exhibited staining that was focally weakly positive for 5-LO reactivity. There were three patterns of cytoplasmic staining observed. The main pattern was a diffuse granular staining, and the other more rare patterns included immunopositivity limited to the free luminal borders of the cells in papillae, producing a line along the outer margin of the papillae without any staining in the perinuclear cytoplasm or that close to the central blood vessels, and dot-like or globular cytoplasmic immunopositivity Image 3A, Image 3B, and Image 3C. In addition, circumferential membranous staining was observed in one case of CPC Image 3D. As a control for the choroid plexus tumor tissue samples, two samples of normal choroid plexus attached to the catheters from two ventricular shunt operations were also analyzed for 5-LO staining. Weak focal staining (0) was observed in the normal choroid plexus tissue of one control, with a cytoplasmic staining pattern that was identical to that observed in the CPP patients Image 2A and Image 2B. Thus, the choroid plexus tumors had significantly more cytoplasmic immunoreactivity than the other central nervous system (CNS) tumors (P < .001).
Immunostains for 5-lipoxygenase (5-LO) in nonneoplastic choroid plexus and in choroid plexus neoplasms. A, Normal choroid plexus tissue attached to the catheter from ventricular shunt operation (H&E, ×200). B, This sample has no immunoreactivity for 5-LO (×400). C, A choroid plexus papilloma (H&E, ×200). D, The same tumor has strong cytoplasmic 5-LO immunopositivity with no nuclear immunoreactivity (×400). E, An atypical choroid plexus papilloma (H&E, ×200). F, This tumor has strong cytoplasmic 5-LO immunoreactivity (×400). G, Choroid plexus carcinoma (H&E, ×200). H, A 5-LO immunostain of this tumor has diffuse strong cytoplasmic immunoreactivity (×400).
Patterns of 5-lipoxygenase immunoreactivity in tumor cells of the choroid plexus (×1,000). A, Diffuse, strong cytoplasmic immunoreactivity. B, Paranuclear dot-like to globule-like immunoreactivity. C, Immunopositivity limited to the free luminal borders of the cells in papillae, producing a line along the outer margin of the papillae without any staining in the perinuclear cytoplasm or that close to the central blood vessels. D, Circumferential membranous immunoreactivity.
Discussion
Human 5-LO is a non–heme iron that contains dioxy-genase.12 Its gene spans 82 kb and includes 14 exons.13 The most characteristic function of 5-LO in the biosynthesis of LTs is the conversion of arachidonic acid and other fatty acids into bioactive compounds. There is mounting evidence suggesting that 5-LO plays a role in tumorigenesis and may be a potential therapeutic target and biomarker.7,14 Ishii et al9 suggested that the 5-LO–LTA4 pathway might play a role in the proliferation of human glioma cells because 5-LO protein immunoreactivity has been observed in various types of human brain tumors, and partial suppression of human glioma cell growth was achieved using inhibitors of the 5-LO–LTA4 hydrolase pathway. Recent studies based on immunohistochemistry have suggested an increased immunoreactivity of 5-LO during high-grade astrocytoma and meningioma.8,15,16
5-LO immunoreactivity is found in the nucleus of certain cell types and in the cytoplasm of others.17,18 These subcellular distributions are not fixed, and translocation of this molecule can result in cellular activation. The nuclear localization of 5-LO usually correlates with increased LT synthesis.19 Previous reports have observed nuclear 5-LO immunoreactivity in various human brain tumors,9,14,20 including astrocytoma, glioblastoma, oligodendroglioma, meningioma, and craniopharyngioma. In the present study, we found similar results in most samples of CNS tumors, but the level of immunoreactivity did not seem to significantly correlate with the subtype of brain tumor, suggesting that 5-LO activation may be a common feature of tumorigenesis in the CNS.
Recent studies have used immunohistochemistry to show cytoplasmic staining for 5-LO in a subset of cells in CNS tumors.7,9 Researchers have suggested that a decrease in LT C4 synthesis or increase in interleukin 5 could trigger the translocation of 5-LO to the nucleus in human blood eosinophils,21,22 but the mechanism of this translocation between nucleus and cytoplasm in tumor cells remains unclear. In this study, we found that most types of brain tumors, including astrocytic, oligodendrogliomatous, ependymal, embryonal, and meningeal tumors, exhibited predominantly nuclear 5-LO staining, with weakly positive cytoplasmic staining in a small number of cases. All cases of human choroid plexus tumors showed cytoplasmic immunoreactivity, and most (92.6%) cases also exhibited strong immunopositivity (2+–3+), but the normal choroid plexus tissue samples from two ventricular shunt operations showed little to no 5-LO positivity. These findings suggest that strong cytoplasmic immunopositivity for 5-LO plays an important role in the tumorigenesis of choroid plexus tumors. In addition, we observed that these cytoplasmic staining patterns were variable. Three of these positive cytoplasmic staining patterns included a diffuse granular staining, immunopositivity limited to the free luminal borders of the cells in papillae, and dot-like or globular cytoplasmic immunopositivity. The significance of these patterns remains unknown.
According to the WHO classification of tumors of the CNS, primary tumors of the choroid plexus can be classified as CPP (grade I), atypical CPP (grade II), and CPC (grade III).11 Choroid plexus tumors express cytokeratins, vimentin, podoplanin, transthyretin, and synaptophysin, and they often also express glial fibrillary acidic protein and S-100 protein.11 Recent studies have found strong immunoreactivity for the inward rectifier potassium channel Kir7.1 and stanniocalcin 1 in normal and neoplastic choroid plexus, and they appear to be highly specific to this tissue.23 Hasselblatt et al24 suggested that TWIST-1 is highly expressed in choroid plexus tumors and is involved in cell proliferation and invasion. In this study, for the first time, we demonstrated that 5-LO was strongly immunopositive in the cytoplasm of human neoplastic choroid plexus cells and that it showed highly sensitive and specific staining in the other types of brain tumors. We believe that 5-LO could be a novel sensitive diagnostic marker of choroid plexus tumors. Choroid plexus tumors need to be distinguished from a variety of low- and high-grade lesions. For example, CPP may closely resemble nonneoplastic choroid plexus, whereas CPC may resemble anaplastic ependymoma, embryonal tumors, or even embryonal carcinoma. Differentiating between choroid plexus tumors and metastatic carcinomas is a significant issue in rare cases when CPCs arise in adults. Our findings suggested that the anti–5-LO antibody might serve as a sensitive and specific marker for the primary and differential diagnosis of choroid plexus tumors. As previously noted, the well-recognized strong immunopositivity for 5-LO in various types of malignant tumor cells suggested that 5-LO might act as a tumor-causing element, allowing it to serve as a new anticancer therapy target.5,14,25,26 Therapy based on a 5-LO antagonist may be a potential new approach to treat tumor cells of the choroid plexus.
In summary, we found that 5-LO is highly expressed in tumors of the choroid plexus but absent from nonneoplastic choroid plexus tissue. The observed cytoplasmic staining pattern has not been described in other types of CNS tumors. Therefore, 5-LO may be helpful during the diagnosis of choroid plexus tumors by serving as a highly sensitive marker.
This work was supported by the Natural Science Foundation of Fujian Province, People’s Republic of China (grant 2014J01413).
References