-
PDF
- Split View
-
Views
-
Cite
Cite
Dick G. Hwang, Xiaohua Qian, Jason L. Hornick, DOG1 Antibody Is a Highly Sensitive and Specific Marker for Gastrointestinal Stromal Tumors in Cytology Cell Blocks, American Journal of Clinical Pathology, Volume 135, Issue 3, March 2011, Pages 448–453, https://doi.org/10.1309/AJCP0PPKOBNDT9LB
Close - Share Icon Share
Abstract
Initial diagnosis of submucosal gastrointestinal stromal tumors (GISTs) is often made from material obtained by endoscopic ultrasound–guided fine-needle aspiration (EUS-FNA). Although 95% of GISTs are positive for KIT by immunohistochemical analysis on surgical specimens, we have observed several cases of GIST that were negative for KIT on the cell block but subsequently positive on the surgical resection. DOG1 has been found to be a specific and sensitive marker for GISTs on surgical material. We compared KIT and DOG1 staining in 52 GIST cell blocks and in 44 cell blocks of other intra-abdominal spindle cell neoplasms. We found that DOG1 was the more sensitive marker, with positivity in all 52 GIST cell blocks. KIT was positive in 46 (88%) of the GIST cases, with sensitivity dependent on the FNA method. Both markers were highly specific: KIT was negative in all 44 non-GIST cases, whereas DOG1 showed weak positivity in only 1 leiomyosarcoma.
Gastrointestinal stromal tumors (GISTs) usually occur in the wall of the gastrointestinal tract and are frequently inaccessible to endoscopic mucosal biopsy. Initial tissue sampling is therefore often obtained by endoscopic ultrasound–guided fine-needle aspiration (EUS-FNA).1,2 Accurate diagnosis is critical to guide neoadjuvant therapy and the surgical approach.
Although GIST exhibits characteristic cytomorphologic features on FNA smears, immunohistochemical analysis has a critical role in differentiating GISTs from other intra-abdominal spindle cell neoplasms, such as leiomyoma, leiomyosarcoma, and schwannoma.3,4 KIT is highly specific and sensitive for GIST, with a sensitivity of approximately 95% on surgical material.5,6 KIT has also been found to be a sensitive marker for GIST on cytologic cell blocks.7 However, in clinical practice, we have encountered several cases of suspected GIST that were negative for KIT in cytologic cell blocks Image 1. It is interesting that the subsequent surgical resection specimens in these cases were positive for KIT.
DOG1 (discovered on GIST1; also known as anoctamin 1, or ANO1) was shown by gene expression profiling to be highly expressed in GISTs.8 This gene was subsequently found to encode a calcium-activated chloride channel in the interstitial cells of Cajal,9 which has a critical role in peristalsis by generating slow waves in gastrointestinal smooth muscle.10 Several recent studies have demonstrated the higher sensitivity of antibodies that recognize DOG1 in the diagnosis of GIST compared with KIT in surgical material.5,11,12
We wanted to investigate whether DOG1 would prove to be a useful marker in the diagnosis of spindle cell neoplasms in FNA material. In this study, we compared KIT and DOG1 in the diagnosis of GISTs and other spindle cell neoplasms in cell blocks.
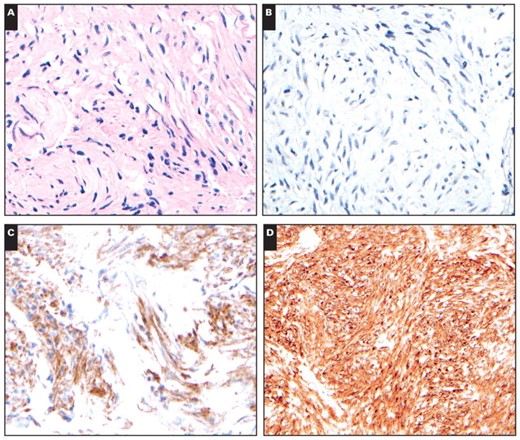
An example of a gastrointestinal stromal tumor in which the initial cell block material (A, ×400) obtained by endoscopic ultrasound–guided fine-needle aspiration was negative for KIT (B, ×400) but strongly positive for DOG1 (C, ×400). Subsequently, the patient underwent a gastric wedge resection, and the surgical specimen was strongly positive for KIT (D, ×400).
Materials and Methods
Cases were retrieved from the files of the Department of Pathology, Brigham and Women’s Hospital, Boston, MA. In total, 52 GIST cytology cases were studied. Cases were included if the diagnosis was confirmed on surgical material (44 cases); or, when no surgical material was available, if the cell block was positive for KIT (7 cases); or, in KIT-negative cases, the cell block was morphologically typical of GIST, positive for CD34, and negative for smooth muscle actin and desmin (1 case). Corresponding material from a surgical biopsy or resection specimen from the same patient was available for comparison in 39 cases. In addition, 44 FNA cases of other intra-abdominal or retroperitoneal tumors in the differential diagnosis of GIST were examined: 25 leiomyosarcomas, 8 leiomyomas, 7 schwannomas, 2 solitary fibrous tumors, 1 desmoid fibromatosis, and 1 endometrial stromal sarcoma. Representative H&E-stained slides and previously performed immunostains were reviewed to confirm the diagnoses.
In our institution, cytology specimens were obtained by EUS-FNA or percutaneous computed tomography (CT)-guided FNA (CT-FNA). EUS-FNA samples were fixed in CytoLyt (Cytyc, Marlborough, MA), and CT-FNA samples were placed in RPMI medium, after which plasma and thrombin were added to the sample to produce a cell block, which was then fixed in 10% formalin before processing for paraffin embedding.
Immunohistochemical studies for DOG1 and KIT were performed on all cases. For DOG1, antigen retrieval was performed in a pressure cooker at pH 6.0. Slides were then incubated with monoclonal anti-DOG1 antibody (clone K9; Novocastra, Newcastle upon Tyne, England) at a dilution of 1:50. For KIT, no antigen retrieval was performed, and slides were incubated with polyclonal anti-KIT antibody (DAKO, Carpinteria, CA) at a dilution of 1:250. Appropriate positive and negative control experiments were performed.
Immunoreactivity was graded semiquantitatively according to the percentage of positive tumor cells: 0, no staining; 1+, fewer than 5% of tumor cells reactive; 2+, 5% to 25% of tumor cells reactive; 3+, 26% to 50% of tumor cells reactive; and 4+, more than 50% of tumor cells reactive. The extent of reactivity was not graded in cytologic cases when only rare isolated tumor cells were present in the cell block. On all cases with reactivity, the intensity of staining was graded as weak, moderate, or strong.
P values to assess significance of differences between sets of counts in ordered categories (eg, staining intensities) were computed by using the nonparametric, 2-tailed Mann-Whitney U test.13
Results
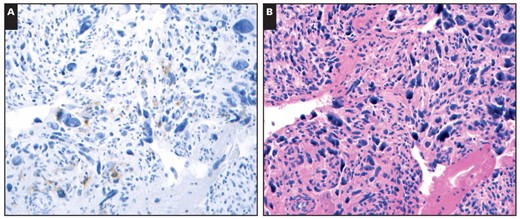
The results of KIT and DOG1 staining in GISTs and other spindle cell neoplasms are summarized in Table 1. Cases with discernible staining in any tumor cells were considered positive. All 52 GIST cell blocks were positive for DOG1, with 47 (90%) showing strong staining. In contrast, 6 (12%) of 52 GIST cell blocks were negative for KIT, and an additional 7 cases showed weak staining. Among the 44 non-GIST cell blocks, no cases demonstrated reactivity for KIT, and only 1 leiomyosarcoma showed weak, focal (2+) staining for DOG1 Image 2. Overall, the sensitivity of KIT for GISTs on cell blocks was 88%, and the specificity was 100%. For DOG1, the sensitivity was 100%, and the specificity was 98%.
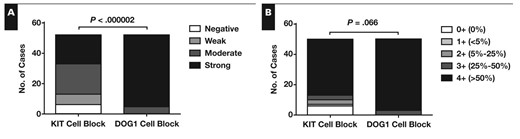
Figure 1 shows the comparison of the staining patterns of KIT and DOG1 in GIST cell blocks. DOG1 demonstrated significantly stronger staining intensity than KIT in cell blocks (P < .000002). A higher percentage of tumor cells was positive for DOG1 than KIT, although this difference was not significant at the .05 level (P = .066). In particular, 47 (94%) of 50 cell blocks demonstrated positivity for DOG1 in more than 50% of the tumor cells, whereas 37 (74%) of 50 cell blocks were positive for KIT in more than 50% of tumor cells. Of note, 2 of 52 GIST cell blocks contained only rare isolated tumor cells and were not graded by extent of staining.
Immunohistochemical Staining for KIT and DOG1 in Intra-abdominal Spindle Cell Neoplasms*
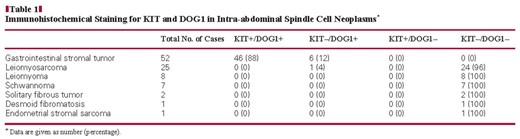
Immunohistochemical Staining for KIT and DOG1 in Intra-abdominal Spindle Cell Neoplasms*

A, DOG1 showed weak, focal staining in 1 of 25 leiomyosarcoma cell blocks (×400). B, The H&E stain demonstrated marked nuclear pleomorphism (×400), which would be highly unusual for a gastrointestinal stromal tumor.
Intensity (A) and extent of reactivity (B) for KIT and DOG1 in gastrointestinal stromal tumor cell blocks. Extent of reactivity was graded by percentage of positive tumor cells. Two cases contained only scattered rare tumor cells and were not graded for extent of reactivity. The difference between KIT and DOG1 is significant for staining intensity but not for extent of reactivity.
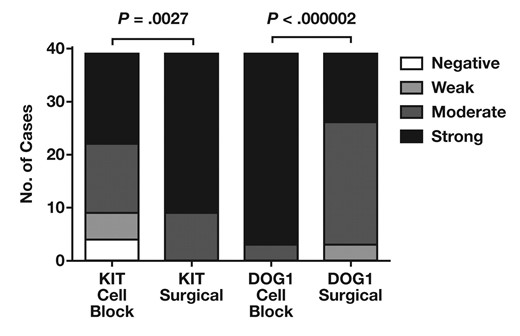
Corresponding surgical material from the same patients was available in 39 GIST cases. Comparison of staining intensity between the surgical material and the cell block Figure 2 showed significantly stronger staining intensity for KIT in the surgical material compared with cell blocks (P = .0027). Among the 4 cell blocks that were negative for KIT, 2 demonstrated weak staining and 2 showed strong reactivity for KIT on the corresponding surgical material. In contrast, the intensity of staining for DOG1 was greater in cell blocks than in surgical material (P < .000002).
Comparison between staining intensity of KIT and DOG1 in gastrointestinal stromal tumor cell blocks and surgical specimens. The difference in staining intensity between cell blocks and surgical material is significant for KIT and DOG1.
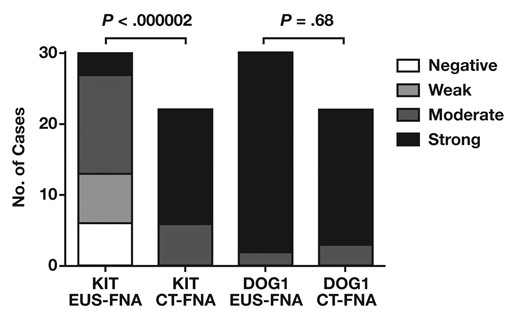
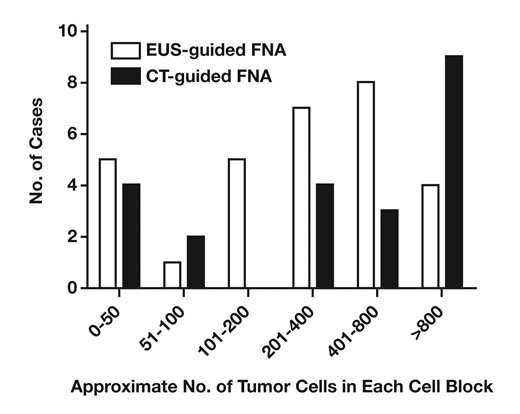
To investigate further the differences in staining patterns, cell block cases were divided between those obtained by EUS-FNA and those obtained by CT-FNA Figure 3. The staining intensity for KIT was significantly lower in EUS-FNA cases than in CT-FNA cases (P < .000002). All 6 cell blocks negative for KIT were obtained by EUS-FNA, and all CT-FNA cases exhibited at least moderate staining for KIT. In contrast, no significant differences in staining intensity for DOG1 were observed between EUS-FNA and CT-FNA cases (P = .68). The number of tumor cells was estimated for each cell block; however, no significant differences were found between the number of tumor cells obtained by EUS-FNA and CT-FNA Figure 4 (P = .22).
Staining intensity of KIT and DOG1 in gastrointestinal stromal tumor cell blocks according to fine-needle aspiration (FNA) method. The difference in staining intensity between endoscopic ultrasound–guided (EUS) FNA and computed tomography–guided FNA (CT-FNA) is significant for KIT but not for DOG1.
Discussion
In this study, we have shown that DOG1 is a more sensitive marker for GISTs than KIT in cytologic cell blocks, whereas both markers are highly specific. Cell blocks also tended to show a higher percentage of cells positive for DOG1 compared with KIT. On cell blocks with limited cellularity, this could lead to a higher rate of false-negatives with KIT compared with DOG1 owing to sampling.
There was 1 leiomyosarcoma case that demonstrated weak, focal cytoplasmic positivity for DOG1. However, given the marked nuclear pleomorphism and unequivocal smooth muscle marker expression in this case, it would be unlikely to be mistaken for a GIST. Positive staining for DOG1 in rare cases of leiomyosarcomas has been observed.11
The sensitivity of KIT for GISTs appears to be dependent on the FNA method, with a significantly lower sensitivity with EUS-FNA compared with CT-FNA. This is unlikely to be due to sample size because we observed no significant differences between the numbers of tumor cells obtained by each approach. There are, however, differences in sample preparation methods at our institution. For CT-FNA, the interventional radiologist generally requests an adequacy evaluation, and the cytotechnologist performing the evaluation places the remaining sample in RPMI medium before the sample is subsequently processed into a cell block following 10% formalin fixation. For EUS-FNA, the endoscopist typically does not request such an adequacy evaluation and places the sample directly in CytoLyt before the specimen is sent to the cytopathology laboratory for processing into a cell block. CytoLyt is a methanol-based fixative that, like alcohol-based fixatives in general, coagulates proteins rather than cross-linking them as does formalin fixation.14 Several markers such as S-100 protein, Hep Par-1, and gross cystic disease fluid protein have been known to show altered reactivity after alcohol fixation.14 The lower sensitivity of KIT in the EUS-FNA may, therefore, be due to this difference in fixation. In general, caution should be used when extrapolating immunohistochemical data based on formalin fixation to other fixatives.15 Given this result, it may be advisable to avoid CytoLyt and other alcohol-based fixatives on submucosal biopsy specimens in which GIST would be a serious diagnostic consideration.
Differences in the rate of KIT protein expression in cell blocks have been noted in the literature, ranging from 75% to 100%.16,17 A recent study examining immunohistochemical staining in GIST cell blocks obtained by EUS-FNA found reactivity for KIT in 36 of 37 cases.7 These results may initially seem to contradict our finding of lower sensitivity of KIT in cell blocks obtained by EUS-FNA. However, the investigators’ method for processing cell blocks from EUS-FNA samples does not involve alcohol-based fixation. Another study also reported a case of GIST that was negative for KIT on cell block but positive on subsequent surgical resection18; this may also be due to an issue of differences in processing, although details of the cell block fixation methods were not provided.
Approximate number of tumor cells per cell block in endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) and computed tomography (CT) guided-FNA cases. No significant difference in numbers of tumor cells was seen between cell blocks obtained by EUS-guided FNA and CT-guided FNA.
We have demonstrated that DOG1 is a more sensitive and robust marker than KIT on GIST cell blocks, and its sensitivity does not depend on the method of processing FNA samples. DOG1 is a useful marker in the diagnosis of GIST on cytologic specimens, particularly when the sample has been fixed in an alcohol-based fixative.
Upon completion of this activity you will be able to:
apply immunohistochemical stains to aid in the diagnosis of gastrointestinal stromal tumors (GISTs).
describe the biologic function of the DOG1 protein.
discuss the relative strengths of DOG1 and KIT staining in the diagnosis of GISTs in cell blocks.
The ASCP is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The ASCP designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit ™ per article. This activity qualifies as an American Board of Pathology Maintenance of Certification Part II Self-Assessment Module.
The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Questions appear on p 479. Exam is located at www.ascp.org/ajcpcme.
References
Author notes
Presented in part at the 99th Annual Meeting of the United States and Canadian Academy of Pathology; March 20–26, 2010; Washington, DC.